bitumen emulsion article

GREEN STAR BITUMEN EMULSION ARTICLE
Foreword
Bituminous emulsions are complex fluids. Their stability is governed by intermolecular forces—a result of a balance of repulsive and attractive forces. The formulator skillfully must understand and balance these forces such that the emulsion can be produced consistently, stored, pumped, transported, and applied by the practitioner in the field without experiencing any downtime in the operation. Bituminous emulsions form the basis for many paving applications in our asphalt industry, including driveway sealants, cold-pour crack sealants, and roofing emulsions. Their rheological (i.e., flow) properties often dictate the uses for which they are suitable. For example, the viscoelastic properties of a slow setting versus a rapid-setting emulsion are different. We expect one day to be able to use rheological properties of bituminous emulsions to predict their success or failure in their respective applications. Even when rheological properties are not critical in the final product, they influence the workability of the emulsion as it is applied in the field. This is true for fog or chip sealing emulsions. Significant improvement has occurred over the years to make quality bituminous emulsion products and their subsequent application in the field. However, there remains considerable work to be done in the sense that further improvement can only occur when the principles of chemistry and physics are fully incorporated into the practical engineering component of road building. The study of bituminous emulsion in our industry can only become less of an intellectual backwater when we begin to incorporate the new advances in experiment and theory of colloid science to create a renaissance in bituminous emulsions in our paving industry. Bituminous emulsions were discussed in a technical session at the 84th Annual Meeting of the Transportation Research Board (TRB). The papers in this document were written following the session and are based on the presentations; the papers in this circular have not undergone a formal peer review. The four papers serve as an overview of the chemistry, production, quality assurance testing, and application of bituminous emulsions. They offer the beginner a start in this exciting and challenging field of bituminous emulsions. Appreciation is expressed to the authors for their contributions; to Robert McGennis, who facilitated the first TRB bituminous emulsion technology session; and to Rebecca McDaniel, who provided valuable editorial input to the text.
Overview of Asphalt Emulsion
The use of asphalt emulsions began in the early part of the 20th century. Today 5% to 10% of paving-grade asphalt is used in emulsified form, but the extent of emulsion usage varies widely between countries. The United States is the world’s largest producer of asphalt emulsion. The advantages of asphalt emulsion compared to hot asphalt and cut back binders are related to the low application temperature, compatibility with other water-based binders like rubber latex and cement, and low-solvent content. The paper gives an introduction to the chemistry of asphalt emulsion. The role of the emulsion components—asphalt, emulsifiers, acids or alkalis, and additives—in determining the physical properties and reactivity of the emulsion is described. Recent advances in the understanding of the setting process are outlined. The classification of emulsions into grades according to their reactivity, particle charge, and physical properties is explained and typical recipes of various emulsion grades are given. The selection of the correct emulsion grade for the various applications based on emulsion reactivity and physical properties of the emulsion is covered in general terms. The past 20 years have seen considerable progress in the understanding of how emulsion chemistry influences performance. Consequently, formulations can be developed to optimize the performance of the construction material or construction process rather than simply to meet standard specifications. The result has been faster-setting surface treatments, quick-drying tack coats, penetrating emulsion primes that are superior to cut backs, and cold-mixed materials with improved properties.
USE OF ASPHALT EMULSION
The first asphalt (bitumen) emulsions used in road construction were prepared in the early part of the 20th century. Today approximately 3 million tons of emulsions are produced in the United States representing about 5% to 10% of asphalt consumption. More than 8 million tons of emulsions are produced worldwide. Emulsion production varies greatly among countries with the United States, France, Mexico, and Brazil being significant producers (1).
ADVANTAGES OF EMULSION
With viscosities in the range 0.5–10 Poise at 60°C, asphalt emulsion is of considerably lower viscosity than asphalt itself (100–4,000 Poise), allowing it to be used at lower temperature. Low- temperature techniques for construction and maintenance reduce emissions, reduce energy consumption, avoid oxidation of the asphalt, and are less hazardous than techniques using hot asphalt. They are also more economical and environmentally friendly than cold techniques using cut back asphalts. The environmental benefit of asphalt emulsion is particularly positive when used for in-place or on-site techniques which avoid the energy usage and emissions associated with heating, drying, and haulage of aggregate. The construction of a roadway with cold techniques has been calculated to consume approximately half the energy of one of similar bearing capacity made with hot-mix asphalt (HMA) (2). An environmental impact analysis (EIA) technique called “eco-efficiency” has been applied to emulsion maintenance techniques (micro- surfacing and chip seal) and it was concluded that the emulsion system had less environmental impact than a thin hot-mix overlay (3). Emulsions are water-based and in many cases can be diluted further with water for applications such as dust control and priming. They are also compatible with hydraulic binders like cement and lime as well as water-based polymer dispersions like natural and synthetic latex. When mixtures of cement, latex, and asphalt emulsion cure, a composite binder is produced with a structure that cannot be duplicated with hot asphalt and with significantly improved properties compared to pure asphalt (4,5).
EMULSION DEFINITION
An emulsion is a dispersion of small droplets of one liquid in another liquid. Typical examples include such everyday products as milk, butter, mayonnaise, and cosmetic creams. Emulsions can be formed by any two immiscible liquids, but in most emulsions one of the phases is water. Oil-in-water (O/W) emulsions are those in which the continuous phase is water and the disperse (droplet) phase is an “oily” liquid. Water-in-oil (W/O) “inverted” emulsions are those in which the continuous phase is an oil and the disperse phase is water. Emulsions can have more complex structures. In multiple emulsions, the disperse phase contains another phase which may not have the same composition as the continuous phase (Figure 1).
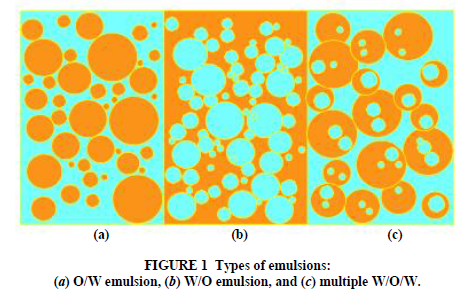
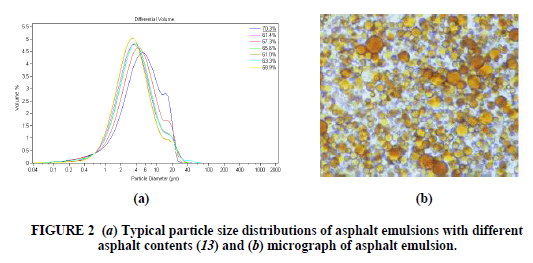
Standard bitumen (asphalt) emulsions are normally considered to be of the O/W type and contain from 40% to 75% bitumen, 0.1% to 2.5% emulsifier, 25% to 60% water plus some minor components which are described below. The bitumen droplets range from 0.1–20 micron in diameter. Emulsions with particle sizes in this range are sometimes referred to as macro- emulsions. They are brown liquids with consistencies from that of milk to double cream, which depend mostly on the bitumen content and the particle size. Some bitumen droplets may contain smaller water droplets within them; a better description of asphalt emulsion would be a W/O/W multiple emulsion. The viscosity of the emulsion and especially changes in the viscosity of the emulsion during storage are strongly influenced by this internal water phase (6,7). There is a distribution of particle sizes in the emulsion, and this distribution is influenced by the emulsion recipe and the mechanics and operating conditions of the emulsion manufacturing plant (Figure 2). The particle size and the particle size distribution of the emulsion droplets strongly influence the physical properties of the emulsion, such as viscosity and storage stability; larger average particle size leads to lower emulsion viscosity, as does a broad or bimodal particle size distribution (8). Particle size also influences the performance of emulsion. In general, smaller particle size leads to improved performance in both mix and spray applications (9). Some recent developments in asphalt emulsion technology have focused on the ability to control the particle size and size distribution of the emulsion during the emulsification process, and consequently to influence the emulsion properties (10–12). Macro emulsions are inherently unstable. Over a period of time, which may be hours or years, the asphalt phase will eventually separate from the water. Asphalt is insoluble in water, and breakdown of the emulsion involves the fusion of droplets (coalescence) (Figure 3). The asphalt droplets in the emulsion have a small charge. The source of the charge is the emulsifier, as well as ionisable components in the asphalt itself. These small charges on the droplets normally provide an electrostatic barrier to their close approach to each other (like charges repel). However, when two droplets do achieve enough energy to overcome this barrier and approach closely then they adhere to each other (flocculate). This flocculation may sometimes be reversed by agitation, dilution, or addition of more emulsifier. Over a period of time the water layer between droplets in a floccule will thin and the droplets will coalesce.
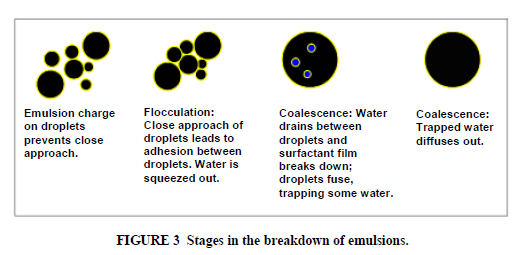
The coalescence cannot be reversed. Factors which force the droplets together such as settlement under gravity, evaporation of the water, shear or freezing will accelerate the flocculation and coalescence process, as does anything which reduces the charge on the droplets. Lower viscosity asphalts coalesce more rapidly than high viscosity asphalts. Of course, eventually we want the emulsion droplets to coalesce after the asphalt emulsion has come in contact with the aggregate and been placed on the roadway. Setting and curing of emulsion are discussed in more detail below.
CLASSIFICATION AND NAMING OF EMULSIONS
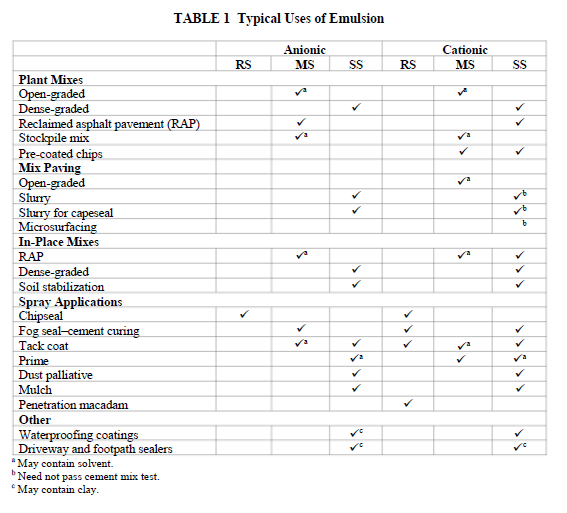
Bitumen emulsions are classified according to the sign of the charge on the droplets and according to their reactivity. Cationic emulsions have droplets which carry a positive charge. Anionic emulsions have negatively charged droplets. Rapid-setting (RS) emulsions set quickly in contact with clean aggregates of low-surface area, such as the chippings used in chip seals (surface dressings). Medium-setting (MS) emulsions set sufficiently less quickly that they can be mixed with aggregates of low surface area, such as those used in open-graded mixes. Slow- setting (SS) emulsions will mix with reactive aggregates of high surface area. RS emulsions are reactive and are used with unreactive aggregates; SS emulsions are unreactive and are used with reactive aggregates. The actual setting and curing time in the field will depend on the technique and materials being used as well as the environmental conditions. In the naming of emulsions according to ASTM D977 and D2397, cationic RS, cationic MS, and cationic SS emulsions are denoted by the codes CRS, CMS, and CSS, whereas anionic emulsions are called RS, MS, and SS, followed by numbers and text indicating the emulsion viscosity and residue properties. For example, SS-1H would be a slow-setting (i.e., low reactivity) anionic emulsion with low viscosity and a hard asphalt residue. CRS-2 would be a reactive cationic emulsion of high viscosity. The QS (quick-setting) and CQS (cationic quick- setting) designations for quick-setting emulsions have been introduced for emulsions intermediate in reactivity between MS and SS, which do not need to pass the cement mix test, and are used primarily in quick-set slurry surfacing applications. Local authorities have many other naming schemes associated with emulsions with particular properties. In state department of transportation (DOT) specifications letters such as P or LM may indicate polymer-modified or latex-modified asphalt emulsion, S may indicate high solvent content, and terms such as AEP (asphalt emulsion prime) and PEP (penetrating emulsion prime), and ERA (recycling agent emulsion) may indicate emulsions with specific uses.
TESTING EMULSION
Emulsion testing will be addressed in detail in another paper. Most test methods have been accepted as ASTM standards. The tests fall into three groups: those that test the handling properties of the emulsion, such as residue content, viscosity, and storage stability sieve residue; those that classify the emulsion into rapid-, medium-, or slow-setting grades, such as demulsibility, cement mix test, and coating tests; and tests on the residue recovered by evaporation, such as penetration or ductility. The emulsion may then be subject to additional performance tests related to the particular application of the emulsion in cold mix, chip seal, etc., using job aggregates.
EMULSION APPLICATIONS
Applications will be addressed in detail in other papers. Some typical applications of the various grades of emulsion are summarized in Table 1, but local practice varies considerably. The choice of emulsion for each application is a question of matching the reactivity of the emulsion with the reactivity of the aggregate and the environmental conditions. Aggregate reactivity is mostly associated with the very finest-size fractions which make the highest contribution to surface area. So a reactive RS emulsion is used with the low-surface area unreactive aggregates used in chip seal, whereas a low-reactive SS emulsion would be used for a dense cold mix which has a high content of –75-micron material and consequently high reactivity. Environmental conditions also have to be taken into account. High temperatures accelerate the chemical reactions and physical processes involved in emulsion setting, and therefore demand slower setting emulsions.
EMULSION APPLICATIONS
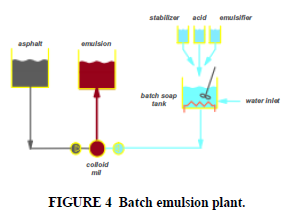
Emulsions are made by mixing hot bitumen with water containing emulsifying agents and applying mechanical energy sufficient to break up the bitumen into droplets. The effect of manufacturing variables on emulsion properties will be described in detail in a later paper. It is clear that the manufacturing process can not only affect the physical properties of the emulsion but also affects the performance of the emulsion. Emulsification is opposed by the internal cohesion and viscosity of the bitumen and the surface tension of the droplet which resists the creation of new interface. Smaller droplets are favored by a high energy input, a low bitumen viscosity at the emulsification temperature and by the choice and concentration of emulsifier (which reduces the interfacial tension). In the most common process, the emulsifier is dissolved in the water phase of the emulsion, and this water solution or “soap” is mixed with the hot liquid asphalt in a colloid mill (Figure 4).
CHEMICAL NATURE OF EMULSIFIERS
Water molecules at the interface between oil and water have higher energy than those in bulk water. The result is an interfacial energy or tension which acts to minimize the interfacial area. The production of emulsion involves the creation of a large interfacial area between the asphalt and water, approximately 500 m 2 per liter (10). Emulsifiers are surface active agents (surfactants).
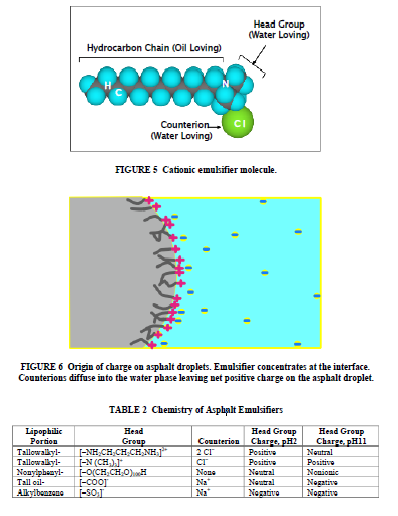
Surfactants have nonpolar lipophilic (oil-loving) and polar hydrophilic (water-loving) portions in the same molecule (Figure 5). The molecules concentrate at the interface between water and bitumen, orientated with the polar group in the water and the nonpolar parts of the molecule in the oil (Figure 6). This both reduces the energy required to emulsify the asphalt and prevents coalescence of the droplets once formed. The choice and concentration of emulsifier also largely determines the charge on the asphalt droplet and the reactivity of the emulsion produced. A typical emulsifier has a hydrophilic “head” group and lipophilic (hydrophobic) hydrocarbon “tail” comprising 12 to 18 carbon atoms. This hydrocarbon tail is represented by “R” in chemical formulas. It is most often derived from natural fats and oils, tall oil, wood resins, or lignin. Emulsifiers can be classified into anionic, cationic, and nonionic types depending on the charge their head groups adopt in water, although this charge may depend on pH (Table 2). Cationic emulsifiers are ammonium compounds contain positively charged nitrogen (N) atoms in their head group; anionic emulsifiers typically contain negatively charged oxygen (O) atoms. The charge on the head group is balanced by a counter ion. The charge on the emulsifier head group largely determines the charge on the asphalt droplets, since the counter ion diffuses away from the asphalt surface (Figure 6). Several studies have shown that even nonionic emulsifiers produce emulsions whose droplets have a small negative charge in water (14), and nonionic emulsifiers are often used in slow-setting asphalt emulsions. In the case of asphalt emulsions, the asphalt itself contains surface active species which can also concentrate at the interface (15). The size and sign of the charge on the asphalt droplets can be measured and is expressed as the “zeta potential” of the droplet. The zeta potential is strongly pH-dependent both because of the pH dependence of the charge on the emulsifier and also because polar components of the asphalt itself may ionize. Zeta potential measurements show that the charge on the asphalt droplets becomes more negative as the pH rises. As the concentration of the emulsifier increases, the particle size of the emulsion is reduced. SS emulsions, which contain higher concentrations of emulsifier, generally have smaller particle size than RS grades.
Generally, more emulsifier is required to provide good stability and the right performance properties to the emulsion than is necessary to fill the interface, so asphalt emulsions will contain some “free” emulsifier in the water phase, present partly in micellar form, which acts as a reservoir helping to prevent coalescence after emulsification and storage and transport. This free emulsifier plays an important role in the setting process (see below) (10, 11). During storage of asphalt emulsion after production there may be slow changes due to migration of polar asphalt components and adsorption of emulsifier from the water phase into the interface (16). This reduces the free emulsifier concentration in the water phase and can influence the emulsion properties.
FORMULATION OF THE EMULSION
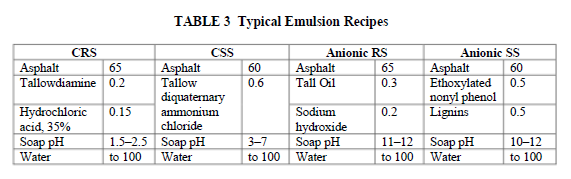
Emulsifiers are often supplied in a water-insoluble form to the emulsion producer and need to be neutralized with acid or alkali by the emulsion manufacturer to generate the anionic or cationic water-soluble form used to prepare the soap solution. The choice of the acid or alkali and the final pH of the emulsion influence the emulsion properties. Hydrochloric acid and occasionally phosphoric acid are the acids used, and sodium and potassium hydroxide are the most common alkalis. Cationic emulsions are usually acid, and anionic emulsions are typically alkaline (Table 3).
RNH + HCl = RNH + Cl
insoluble form water-soluble cationic form
RCOOH + NaOH = RCOO + Na + H O
insoluble form water-soluble anionic form
Some emulsifier types, like quaternary ammonium compounds and alkylbenzenesulphonates, have permanent head group charges and do not need to be reacted with acids or alkalis (Table 2). These products, as well as nonionic emulsifiers, allow the formulation of emulsions which are neutral in pH. Increasing emulsifier concentration decreases the reactivity of the emulsion. MS emulsions are generally formulated with the same emulsifiers as RS grades but at higher concentration (0.4%–0.8%). The emulsion producer can adapt the emulsion recipe to cope with reactive aggregates or high temperatures, generally by increasing the emulsifier concentration or blending emulsifiers of lower reactivity.
A wide range of chemistries have been used for asphalt emulsifiers. In addition to cationic, nonionic, and anionic emulsifiers, there are products with amphoteric head group character which may adopt positive or negative charges depending on pH.
OTHER COMPONENTS AND THEIR FUNCTION
Calcium and Sodium Chlorides
Asphalt contains a small amount of salt, which can lead to an osmotic swelling of the droplets in an emulsion as water is drawn into the droplet. This results in an increase in emulsion viscosity, often followed by a decrease as the salt slowly escapes. Calcium chloride or sodium chloride is included in the emulsion at 0.1%–0.2% to reduce the osmosis of water into the bitumen and minimize the changes in viscosity. Sodium chloride is used in anionic emulsions (6).
Adhesion Promoters
Water resistance is an important property of asphalt mixes and seals. The cured film from some anionic emulsions and occasionally also cationic emulsions may not have sufficient adhesion to aggregates, in which case adhesion promoters can be added to the asphalt or to the finished emulsion. Generally, these adhesion promoters are surface active amine compounds.
Solvent
Solvents may be included in the emulsion to improve emulsification, to reduce settlement, improve curing rate at low temperatures, or to provide the right binder viscosity after curing. The maximum amount of distillate in the emulsion is usually specified. MS emulsions often contain up to 15% solvent to provide the right workability characteristics and stockpile life to asphalt mixtures. Emulsions used in recycling may also contain solvents and fluxes in order to rejuvenate old asphalt.
Latex
Polymer modification can improve the properties of bitumen in terms of cohesion, resistance to cracking at low temperatures, and resistance to flow at high temperatures. Latex is a water-based dispersion of polymer which is particularly suited to the modification of emulsions. Latex comes in anionic, nonionic, and cationic forms, and it is important that the latex type should be compatible with the emulsion. Styrene butadiene rubber, polychloroprene, and natural rubber latex are most commonly used in paving grade emulsions.
THE SETTING PROCESS
Emulsified asphalt must revert to a continuous asphalt film in order to act as cement in road materials. This involves flocculation and coalescence of the droplets and removal of the water (Figure 3). Evaporation and absorption of water by the aggregate may be the main breaking mechanism for very slow-setting emulsions, but in most cases chemical reactions between the aggregate and the emulsion contribute to the emulsion setting and it is not necessary for all the water to evaporate before curing takes place. The strength of the reaction of emulsion with aggregate is in many cases sufficient to squeeze the water from the system. Clean water can be seen separating from the mixture. The speed of these setting and curing processes depends on the reactivity of the emulsion, the reactivity of the aggregate and environmental factors, such as temperature, humidity, wind speed, and mechanical action. Less viscous asphalts tend to give faster coalescence. It may take a few hours in the case of a chip seal to several weeks in the case of a dense cold mix for the full strength of the road material to be reached. A considerable amount of research effort, partly sponsored by the European community, has been expended to elucidate the mechanism of setting and curing of asphalt emulsion (17–19).
Important factors are changes in pH caused by reaction of the aggregate with acids in the emulsion, adsorption of free emulsifier onto the aggregate surface, and flocculation of the emulsion droplets with the fines. The relative timescale of flocculation (setting) and coalescence (curing) depends on the system, but in general flocculation is the more rapid process in which some water can be expelled from the system and some cohesive strength develops, followed by a slower coalescence process which results in a continuous asphalt phase. This asphalt phase must also adhere to the aggregate. Coalescence is an inversion process; the O/W emulsion is transformed into a W/O type which then slowly loses its internal water phase. This inversion process is favored as the ratio of asphalt to water in the system increases. The tendency for an emulsion to invert can be determined in laboratory tests and has been related to curing behavior in the field (21). Aggregates take up a characteristic surface charge in water which depends on the nature of the minerals, the pH, and the presence of soluble salts. So-called “acid” aggregates high in silica tend to take up a negative charge.
Some aggregates, like carbonates, and fillers, like cement, may neutralize acid in cationic emulsions causing the pH to rise and the emulsion to be destabilized. Anionic emulsions may be destabilized by soluble multivalent ions. We can consider two extreme cases of emulsion breaking (17). In the case where the charge on the emulsion droplets is quickly destroyed by pH changes, for example, then the emulsion very quickly flocculates and coalescence begins to occur at a slower rate. This rate is dependent on the viscosity of the binder, as well as environmental conditions; coalescence is slower with high viscosity asphalts and lower temperatures. At the other extreme where the emulsion droplets remain charged, loss of water, either by evaporation or by absorption of water into porous aggregate, eventually forces the droplets close enough for attractive forces to predominate, forcing out water and starting the coalescence process. The attractive forces between the droplets can generate significant cohesion even before coalescence occurs. In a simplified process (Figure 7) of the setting of a RS cationic emulsion where the aggregate does not contain significant fines, important stages in the setting process can be considered as follows:
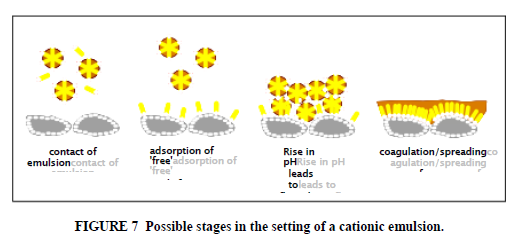
1. Free emulsifier adsorbs onto the (oppositely charged) mineral surface, which neutralizes some charge on the surface while at the same time making the surface somewhat lipophilic. Too high a free emulsifier concentration in relation to the surface area of the aggregate can actually reverse the charge on the minerals and so inhibit the setting of the emulsion.
2. Minerals neutralize acids in the emulsion, causing loss of charge on the emulsion droplets, leading first to flocculation of the asphalt droplets and then to a slower coalescence of the droplets.
3. Water is adsorbed by the mineral, as well as evaporates from the system.
4. Droplets in contact with the mineral spread on the surface, especially that surface made lipophilic by adsorbed emulsifier, eventually displacing the water film on the aggregate surface.
In the breaking of SS grades, where the aggregate contains high content of fines, heteroflocculation of the droplets of asphalt and the oppositely charged fines may occur, which is sufficiently strong to squeeze out water and form an asphalt mastic. A similar situation is achieved in microsurfacing where filler is intentionally added to initiate setting. Mechanical action, such as compaction or traffic, may squeeze the droplets together, promoting coalescence and squeezing water out of the coalesced film. In practical situations too early coalescence of the asphalt droplets can hinder final curing by skin formation (20, 21) reducing the evaporation of water. Coalescence throughout the asphalt emulsion film, before water is trapped in the system, is promoted by smaller asphalt droplets with narrow size distribution. Too early coalescence of asphalt droplets in some systems can interfere with the formation of a composite binder formed from latex and asphalt, which depends on latex curing before asphalt (4).
RECENT DEVELOPMENTS
Emulsion Manufacture
The factors influencing the properties of the emulsion are better understood today. The particle size of the emulsion and the free emulsifier concentration can be determined by the manufacturing conditions and viscosity can also be controlled. High residue ( 75%) emulsions can be prepared by the production of bimodal particle size distributions (22). Production of emulsion by static mixer technology (SMEP = static mixer emulsion process) is fully commercialized and holds the possibility of adjusting the particle size distribution of the emulsion and so controlling physical and performance properties of the emulsion (23).
Emulsion Chemistry
In the area of emulsifier chemistry there continues to be innovation, especially in emulsifiers for SS emulsions. The use of phosphoric acid instead of hydrochloric acid in emulsions for micro- surfacing and cold mix has been established in Europe, Asia, and recently also in the Americas. The phosphoric acid system allows a wider range of asphalts to be used (24).
Setting Process
As mentioned above there has been a tremendous improvement in our understanding of the setting process of asphalt emulsion. Some innovative techniques related to the setting process have included the use of breaking agents in cold mix and spray applications (25, 26) to provide accelerated curing; the use of wetting agents as compaction aids in cold mix (27) to allow quicker removal of water and hence earlier bonding of the coalesced asphalt with the mineral surface; cement-free slurry seal systems in which reactive filler is generated chemically in the slurry (28); and emulsions of mixed hard and soft binders for cold mix applications which allow some control over the coalescence process (29). In some novel processes SS and RS emulsions are used in combination in order to provide the right coating and curing behavior.
Overview of Asphalt Emulsion Applications in North America
An estimated 10% of paving-grade asphalt in North America is used in the emulsified form. The advantages of low temperature application, solventless, or low solvent content formulations and easy modification with water-based latex emulsions make asphalt emulsions the material of choice for sealers and binders in many pavement maintenance and construction applications. Although asphalt emulsions have been in use for almost a century, use in specific applications varies significantly throughout North America, with some applications in common use throughout Mexico, the United States, and Canada, and other applications used extensively in some areas and not in others. Since lack of awareness of and exposure to the broad range of construction and maintenance applications for emulsions can determine usage, this paper gives an overview of practical applications carried out with asphalt emulsions for construction, maintenance, and preventive maintenance applications. The purpose of each treatment, the type of emulsion used, and the equipment involved is indicated.
CONSTRUCTION APPLICATIONS
The most common construction applications of asphalt emulsions include the following.
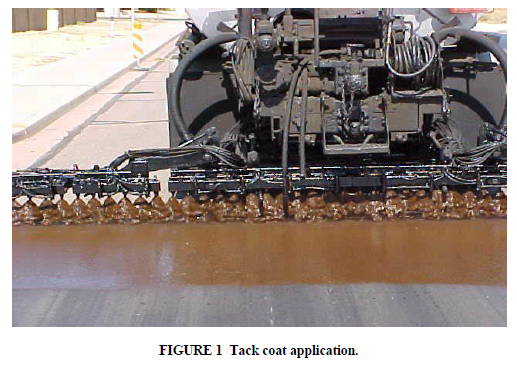
Tack Coat
Purpose
Tack coats are used in construction applications to provide a good bond between the existing surface and a surface treatment (Figure 1).
Types of Emulsions Used
Emulsions used for tack coats can be anionic or cationic, slow-setting emulsions. Tack coats are intended to break quickly (usually within 15 min of application). They have very low application rates (around 0.1 gsy), usually at a dilution rate of one-part emulsion to one-part water. In order to be able to dilute tack coat emulsions to guard against over application and potential bleed through, these emulsions must be sufficiently stable and therefore are slow setting by category. The very thin film application rate results in a fast break. The intent is to ensure a good bond between pavement layers by allaying any dust on the existing surface and wetting existing oxidized surfaces with a bond coat, without adding excess binder to the surface treatment.
Equipment
Tack coats are applied through an asphalt distributor spray bar. Distributors are generally computer controlled to achieve a very accurate application rate.
Emulsion Mixes
Purpose
Emulsion chemistry is such that it enables excellent coating and adhesion of the residual asphalt cement to aggregate surfaces in both dense and open-graded mixes, base stabilization, and stockpiled patching mixes (Figures 2, 3, 4, and 5). Emulsion mixes are economical where small quantities of mix need to be produced at locations remote from hot plants and when ambient temperatures make the use of hot mix problematic.
Types of Emulsion
Slow- and medium-setting, mixing-grade anionic, and cationic emulsions, with and without solvent or adhesion agents, are used for both mixes and base stabilization. Polymer modifiers may be used to control the performance properties of the residual binder.
Equipment
Emulsion mixes can be produced with a wide variety of equipment, either on-site, with in-place pulverizers, mixer/pavers, or at central plants. Both blade- and paver-laid mixes are commonly used. Mixing and laydown equipment is essentially the same as that for hot mix production. Processes vary to accommodate the difference in binders including the following: pavers with a heated screed are not used; delayed rolling may be implemented to accommodate evaporation of excess moisture; lime stabilization and subsequent sanding of solvent containing mixes may be necessary prior to rolling; and rolling patterns may differ significantly from those used for hot mix.



Penetrating Prime
Traditionally penetrating primes were cutback asphalts. With increased knowledge of the chemistry of emulsions and control over the breaking and setting characteristics, solventless emulsions are successfully being used as penetrating primes on compacted base courses to seal and prime the surface prior to surface treatments to ensure adequate bond or as an application for dust abatement. Types of Emulsions Specially formulated penetrating grade, slow-setting cationic and anionic emulsions are used and are designated AEP (asphalt emulsion prime) or PEP (penetrating emulsion prime, generally dilute). Some emulsions for prime coats may still contain low levels of solvents. Equipment Standard, computer-controlled asphalt distributor trucks are used to apply penetrating primes.
PAVEMENT MAINTENANCE APPLICATIONS
Scrub Seals
Purpose Scrub seals are an effective surface treatment for oxidized or distressed pavements (Figure 6). Scrub seals function by sealing fine cracks prior to application of surface chips or by creating a mastic seal on distressed pavements. Type of Emulsion Emulsions for scrub seals may be anionic or cationic, with or without rejuvenating agents and polymer modifiers. Emulsions for scrub seals should be formulated to give adequate time and stability to enable scrubbing of the emulsion into the existing surface prior to chip placement and, when necessary, scrubbing of the chip and emulsion into a distressed pavement. Equipment Scrub brooms are added to conventional chip seal equipment for scrub seal application (Figure 7). Primary scrub brooms are designed to force emulsion (generally polymer modified) into micro cracks in the pavement surface. Secondary scrub brooms designed to force the emulsion– chip blend into fatigue cracks in a distressed pavement may be used also. Technology in design of scrub brooms ensures filling of cracks and uniform application of binder and chip across pavement cross-section without excess binder at the edges of pavement (Figure 8).


Chip Seals
Purpose Chip seals are an effective maintenance tool for restoring a wearing course to a pavement. As a preventive maintenance tool, chip sealing prevents ingress of moisture into a pavement or base course and can prevent deterioration due to oxidative aging of a pavement. Type of Emulsion Emulsions for chip sealing include anionic, cationic, rejuvenating, polymer-modified, and high float formulations. Chip seal binders do not generally contain solvents but some, such as high floats, have a provision for solvent content, as required, to wet chips and ensure adhesion. Because many different geologic types and gradations of cover aggregate are used for chip sealing (single size, graded, hard, porous) the emulsion should be selected to accommodate the aggregate in a performance system. Equipment Chip sealing utilizes computer controlled asphalt distributor trucks, chip spreaders designed and calibrated to deliver accurate quantities of chip, pneumatic rollers, and brooms for removal of excess chip (Figures 9, 10,11). Steel wheeled rollers may be used on multiple application chip seals to “rack-in” aggregate.


Slurry Seals
Purpose
Slurry seals are fine aggregate emulsion mixes that can provide a smooth to moderately textured surface for low-speed, low-traffic volume streets and roads. Slurry seals cure quickly and have the advantage over chip seals of being water-based systems that produce no dust or loose chip during resurfacing. Slurry seals can produce smooth, aesthetically pleasing surface textures similar to hot mix in a very thin (3/8 in.) application (Figure 12).
Type of Emulsion
Slurry seal technology has advanced over the past 40 years from slow-setting systems using anionic emulsions. These systems were dependent on atmospheric conditions for adequate curing by evaporation before the surface could be opened to traffic. Current slurry seal technology provides for very rapid cure systems based on the use cationic emulsions with and without polymer modification. Slurry technology derives its success from joint development of appropriate emulsions, equipment and additive systems, which combine with well-defined aggregate characteristics to ensure production of a controlled cure mix which resists raveling.

Slurry Seals
Equipment
Sophisticated slurry machines are on-grade traveling production plants that provide storage and mixing capability for aggregate, emulsion binder, water for consistency control and additives, which may be used for control of breaking and setting characteristics. Slurry machines include a pull-along laydown box for application of the slurry to the roadway (Figure 13). Laydown boxes are equipped with augers to ensure uniform mixing and application and to prevent premature breaking of the mix prior to placement.
Microsurfacing
Purpose
Microsurfacing is state of the art resurfacing of pavements with a thin layer emulsion mix designed to withstand heavy traffic. Like slurry sealing, microsurfacing produces no dust or loose chip. Microsurfacing design enables rapid cure of the surface regardless of the depth of placement and is therefore an excellent treatment for rutted or irregular surfaces.
Types of Emulsion
Microsurfacing emulsions are specially formulated cationic emulsions designed to work with a specific aggregate in a performance mix composition. The emulsion formulation is tailored to the aggregate characteristics to produce a mix which must comply with stringent specifications designed to ensure against rutting, raveling, bleeding, or premature aging. Microsurfacing emulsions are highly polymer modified.
Equipment
Microsurfacing machines are sophisticated travel plants designed to store and mix the aggregate, emulsion, mix water and additives for breaking control. Microsurfacing machines generally have twin shaft mixing chambers specially designed to eliminate dead spots associated with a pug mill. Laydown boxes also have augers for uniform mixing and application. Special boxes have been designed for rut-filling prior to full-width resurfacing (Figures 1316).


MULTIPLE APPLICATION SURFACE TREATMENTS
Double and triple chip seals are commonly used for new construction sealing in rural areas. In addition to these multiple seals, the following multiple application seals are also frequently used.
Cape Seals
Purpose
Cape seals consist of an application of slurry seal or microsurfacing placed over a chip sealed surface. The chip seal is placed to ensure sealing and waterproofing of the existing surface. The slurry or microseal is placed over the chip seal to eliminate the risks associated with loose chips as well as to establish the desired surface texture.
Types of Emulsion
Emulsions for the chip seal portion of cape seals are the same as for regular chip seals. Slurry or microemulsion are most always cationic and generally polymer modified. Equipment
Equipment for both chip and slurry or micro portions of a Cape Seal are the same as for the individual treatments.
Cape Scrub Seals
Purpose
Cape scrub seals are cape seals where the polymer-modified emulsion has been scrubbed into the existing distressed surface prior to chip and slurry or microsealing (Figure 17).
Types of Emulsion
Emulsions for each of the applications are the same varieties as those used for the individual applications.
Equipment
The equipment used for cape scrub seals is the same as that for cape seals with the addition of the emulsion scrub broom used before applying chip.

Ultrathin Bonded Wearing Course
Purpose
An ultrathin bonded wearing course is an application of an asphalt emulsion immediately covered by a thin layer of hot mix (Figure 18). The emulsion is effectively drawn up into the hot mix to form a strong cohesive bond with both the pavement and the hot mix.
Types of Emulsion
Emulsions for ultrathin bonded wearing courses are mostly cationic polymer-modified emulsion formulations.
Equipment
Equipment for this surface treatment is a specially modified paver equipped with an emulsion spray system which places the emulsion on the pavement surface just before the hot mix screed.

PREVENTIVE MAINTENANCE APPLICATIONS
Fog Seals
Purpose
Light applications of anionic or cationic, dilute emulsions, with and without rejuvenating agents and polymer modifiers are applied routinely to pavement surfaces as a preventive or corrective maintenance tool. Fog seals may be applied to virtually any asphalt surface treatment, hot mix or emulsion mix (Figure 19). Fog seals are the most cost-effective preventive maintenance tool and should be considered for routine maintenance programs (Figure 20).
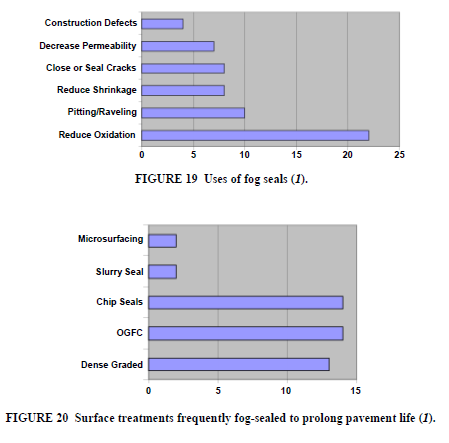
Types of Emulsions
There are numerous anionic and cationic emulsion formulations with and without rejuvenating agents and/or polymer modifiers for use in fog sealing. To minimize the temporary reduction in skid resistance which results immediately after fog seals are applied, light sanding may be carried out (Figure 21).
Equipment
Equipment for application of fog seals are computer-controlled asphalt emulsion distributors. Because of reduction in skid resistance which is aggravated by over application of emulsion, equipment should be accurately calibrated. Sand spreaders may be required if a sand bond breaker is desired to minimize loss of skid resistance.

Recycled Asphalt Pavement Mixes
Purpose
Both new construction and reconstruction are possible utilizing recycled asphalt pavement (RAP) mix technology. Mixes composed of graded RAP millings and state of the art emulsion binders can produce mix properties equal to those of hot mix in dense, gap, and open-graded mixes. These mixes can be produced more economically than hot mix.
Types of Emulsions
Mixing grade emulsions, both anionic and cationic, with and without solvent, are used in RAP mixes. Emulsions may contain rejuvenating agents or polymer modifiers and adhesion agents.
Emulsion chemistry is responsible for immediate coating and subsequent cohesive strength development. Residual binder properties determine long term performance.
Equipment
Equipment for processing and placement of RAP mixes is essentially the same as that for producing emulsion mixes from virgin materials. A wide variety of crushing, sizing, mixing, laydown, and compaction equipment is used.
New construction mixes (placed on compacted base) are generally on-site mixed and hauled to grade for paver laying (Figures 22 and 23). Reconstruction of existing pavements is generally more economical using cold-in-place recycling trains consisting of sizing, crushing, mixing, placement, and compaction equipment on grade (Figure 24).


RAP Millings in Slurry Surfacing
Purpose
RAP millings, when properly screened and graded, provide a cost effective aggregate for slurry surfacing (Figure 25).
Types of Emulsions
Primarily cationic mixing grade emulsions are used in RAP slurry mixes. These emulsion slurry mixes are designed for compliance with all standard slurry mix performance requirements.
Equipment
RAP slurry mixes are placed with standard slurry mixing and application equipment.
SUMMARY
Asphalts in their emulsified form are used in a wide range of applications for construction, maintenance and preventive maintenance treatments. Development of technologies utilizing asphalt emulsions are a product of cooperation among emulsion manufacturers, equipment manufacturers and contracting companies.

The advantages of low-temperature, low-volatile emission application without the safety issues of hot asphalt make emulsions the preferred material in many applications. Asphalt emulsion usage will certainly increase for the above reasons as well as due to efficiencies in use, which will increase with rising fuel and energy costs.

